how does cathodic protection work for ships
Introduction
Corrosion takes away the durability and strength of the metal. Corrosion is a ship’s worst enemy because it just cannot be avoided due to the continuous presence of moisture . Metal is used in the construction of ship and as there is a continuous contact of the ship with water and moisture, the formation of corrosion is a perennial process. So how corrosion can be avoided or minimized?
For this purpose cathodic protection is used and to understand cathodic protection it is necessary to understand the corrosion process in detail. Due to the presence of moisture and water, the materials used in the ship are in a continuous process of reactions. These reactions can be divided into chemical and electrochemical reactions. The occurrence of the chemical reaction is generally at the surface of the metal and it is there where the degradation of the metal starts.
Chemical reactions
Generally the exchange of charge takes place between the reactants at a particular place and what results is known as the local effect and the resulting reaction is called chemical reaction. An example of this is the reaction between the steel surface and atmospheric oxygen. The reaction leads to the formation of oxide layer on the surface of the metal. This is the germinal site of the corrosion process. Now if we take a specific case like iron, then the resulting reaction will lead to the formation of iron oxide and when water or moisture comes in contact, the compounds reacts to give iron hydroxide, also known as rust. Due to the porosity of rust the formation of oxides continues.
Stainless steel is not affected my chemical reaction because the first layer of oxide is not affected by water. This is because there is a lack of oxygen between the metal and the oxide layer which prevents any further development in the oxide layer.
Ship Side Corossion


Electrochemical reactions
Electrochemical reactions always involve compounds that have the capacity to dissolve ions or charged particles in them. These kinds of compounds are acids, bases, some metals and gases. These compounds provide mobility to these ions. Due to this the chemical reactions and the currents are not confined to one place. This results in the spreading and proliferation of the reaction in much larger area.
The type of the metal plays a very important role in the kind of chemical reaction that will take place. All metals when in contact with water have a tendency to form ions. Metals lost ions and become negative and water gain ions and become positive. This means that if the metal is less noble it will give out more ions making itself more negative and water more positive. And if the metal is more noble then it will give out less ions remaining less negative. We can enumerate some important metals in descending manner of their tendency to lose ions.
Gold>copper>tin>iron>zinc>aluminum

Galvanic corrosion and the use of sacrificial elements.
Transfer of ions takes place between two different metals also. When two different metals are in contact with each other in the presence of water then a flow of electric current takes place between them. The flow of electric current or the ions is generally from a metal of higher potential to the one with lower potential. The flow of electrons takes place due to the difference in the potentials of the metals. The metal with lower potential gradually releases more ions to the water. After certain amount of time the metal releases all the ions and fully disappears. The process of releasing ions is known as anodic reaction and the metal that dissolves is known as anode.
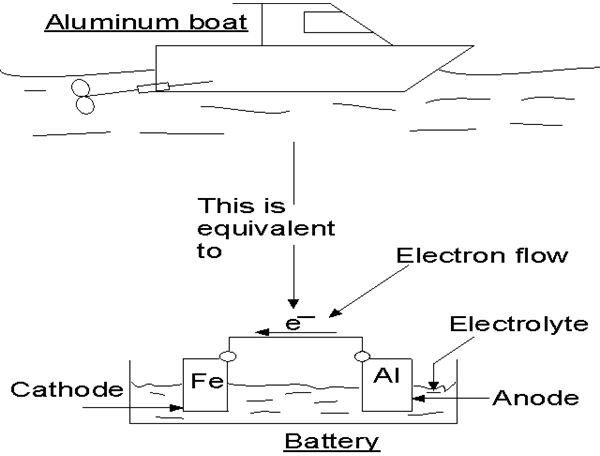
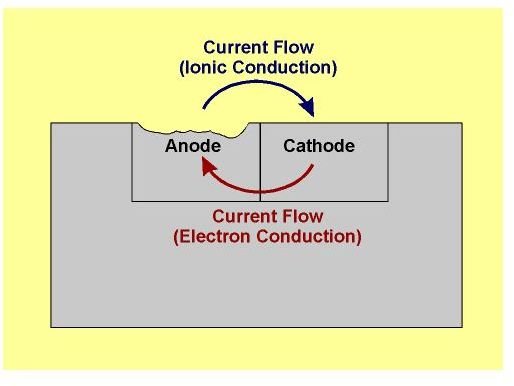
Parts affected on ship
All those metals on ships whose composition is not homogenous become victim of the adverse effects of corrosion. One such substance is brass, which is formed of zinc and copper. Due to contact with moisture, Zinc which is anodic in nature dissolves leaving behind copper. This process goes on until the dissolving of zinc is stopped.
The main areas that get affected on ship are the propeller region, piping system, copper plates of heat exchangers. Aluminum parts that are used in the machineries. And the steel plates in the different sections of the hull. Corrosion also takes place in and around the regions where formation of ice takes place, e.g. refrigeration and air conditioning areas.
Also the parts where there is a wear down due to friction. Activities such as mooring and tugging leads to such wear down. Corrosion also increases due to high speed of the moving part and also due to salinity and temperature of water. Having learnt so much about corossion I am sure you would be interested in knowing about ways to fight corossion on ships.

References
Ship knowledge - A Modern Encylopedia by K. Van Dokkum
Images
https://www.seaguard.co.nz/images/battery.gif
https://www.corrosion-club.com/images/corrosioncell.gif
https://upload.wikimedia.org/wikipedia/commons/9/95/Corrosion-on-ships-bow.JPG
https://www.safetycenter.navy.mil/images/a-m/confined3.jpg
https://www.marinebuzz.com/marinebuzzuploads/EasyWayofPipeSurfacePreparationforPainti_9C41/pipe2.jpg
