Copper Smelting Process - Furnaces Used, By-Products, and Process Used to Smelt Copper
Introduction to Copper Smelting Techniques
Copper ore typically contains only about 0.6% of the actual copper mineral, the most common ore mined being chalcopyrite. The ore is attained using traditional methods of underground and open pit mining, the later accounting for 90% of the ore used in smelting. The ore is crushed, screened, and washed before being further processed ready to supply the smelting furnace.
There are several methods of copper smelting, the popular ones being reverberatory smelting and oxygen flash smelting. Both methods require the resultant molten copper to be further processed to achieve a grade of 99.9% pure copper. During the process, the gas given off is converted to sulphuric acid, which can be used in the plant or sold off as a by-product.
This is another article in my series on the smelting of metals, this one focusing on the smelting of copper. We shall begin by looking at the mining and processing of the copper bearing ore, followed by an examination of an established and a modern copper smelting process.
Mining for Copper Bearing Ore
There are two categories of copper ore; sulphide ore which is processed to enrich it before smelting, and oxide ore that is subjected to acid treatment. The major producers of copper ore are Chile, USA and Australia, the vast majority of the ore coming from open pit mining.
In open pit mining the layers of soil and vegetation seam are scooped away until the copper bearing ore is exposed. This is removed by blasting, the resulting ore collected and transported to the ore processing plant. Here it is crushed, washed and screened to remove most of the gangue.
The finer sulphide ore is then subjected to an ore enrichment process, where the crushed ore is added to a vessel containing pine oil and coalescing agent such as xanthate. Air is injected to the vessel to promote bubbles from the pine oil which, due to the xanthate the fine ore clings to. This concentrated ores float to the top of the vessel where the froth is skimmed off, calcified and crushed to fine ore containing about 25% copper.
Meantime, the coarse oxide ore is subjected leaching before being sent for further processing into copper by solvent extraction.
Sketches of Furnaces and Smelting Process
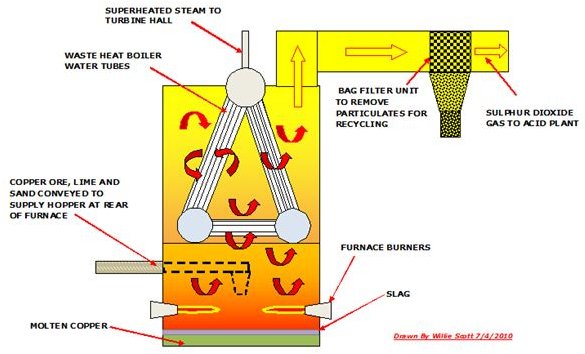
Smelting Copper Using a Reverberatory Furnace
A reverberatory furnace is fabricated from steel beams into a rectangular structure which is lined outside and inside with firebricks. Fossil fuel and/or oxygen fired burners provide the exothermic heat required to melt the copper ore which is supplied by conveyor to a gas tight hopper. It has a tapping point for the molten copper and slag. There is normally a waste heat boiler incorporated in the reverb furnace as a lot of heat is applied in this smelting process.
The furnace is fired and the enriched ore, which can include limestone and sand, is conveyed and fed into the furnace. Here it is subjected to intense heat and becomes molten. This copper which is about 65% purity is then tapped and transferred to a converter furnace, whilst the slag which forms on top of the molten copper is also tapped and either recycled or discarded to a slag heap.
The sulphur dioxide gas produced in the furnace is extracted by centrifugal fans and discharged through ducting to the acid plant, an essential part of every copper smelter.
Here it is processed and converted to sulphuric acid being used in smelting and ore processes, any remaining acid being sold off as a by product.
Meanwhile in the converter furnace, the molten copper is being subjected to an injection of oxygenated compressed air. The oxygen reacts with the sulphide ore, producing copper sulphate whilst converting the copper ions to blister copper of over 98% purity.
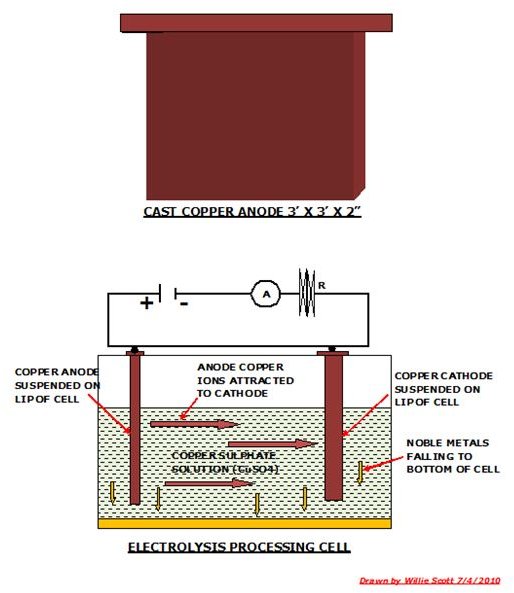
The converter furnace is tilted and the blister copper poured is into a crucible which is transported by overhead crane and tipped into an anode furnace. The molten copper is now tapped and run into 3’ x 3’ moulds from which the copper anodes are formed.
These anodes are now transported to the tank shop where they are subjected to electrolytic refining. This process consists of suspending an anode in a tank containing copper sulphate and a plate of pure copper effectively acting as a cathode. A current is passed through the solution and the copper ions are stripped from the anode and deposited on the cathode, the impurities falling through the solution to the bottom of the tank.
Among these impurities are noble metals such as gold, platinum and silver which are periodically removed along with the sludge from the bottom of the tank. The tank shop houses several hundred such tanks that in a continuous process refine the copper anodes to cathode copper to 99.99% purity.
The cathode copper is now ready for transportation to the various copper extrusion and alloying specialist facilities.
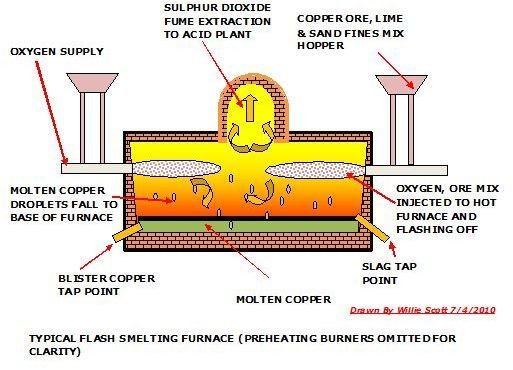
Copper Smelting Using Oxygen Flash Technique
The oxygen flash technique is a relatively new and efficient method of smelting copper. The operation takes place in a furnace which has been preheated preferably by natural gas burners. A fine mix of copper ore, sand and limestone are injected by compressed oxygenated air, prompting immediate endothermic combustion and temperatures of 1100°C, without using any other fuel source. The copper ore become molten copper droplets that fall to the bottom of the furnace forming a molten copper layer with a slag floating on top of it. The molten copper is tapped and sent through the same processes as the reverb smelted copper, except for the converter stage, as it has been already converted to blister copper in the flash furnace.
References and Additional Resources
https://www.elmhurst.edu/~chm/vchembook/335coppersmelter.html