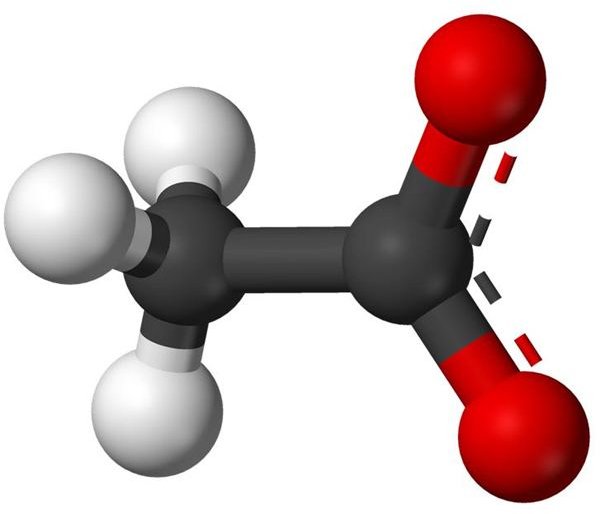
The Chemical Formula for Acetate
Acetates are an extremely useful class of chemicals with a wide array of industrial, pharmaceutical, and biological uses. Acetates are made by chemically modifying acetic acid to produce either salts or esters. A salt is a type of ion formed from the neutralization of an acid by a base, in which each positively charged molecule is balanced by a negatively charged one. Esters are created by reacting acids with molecules containing a hydroxyl group (usually an alcohol).
There are many industrial uses for both acetate salts and esters. Because the different compounds are produced by different chemical reactions, they have different chemical formulas. Read on below to learn the chemical formula of acetates - both acetate salts and esters.
The Chemical Formula of Acetates
Acetates are ultimately formed by removing the acidic hydrogen atom from acetic acid (chemical formula C2H4O2). This reaction results in either an acetate salt or acetate ester, depending on the method used.
Acetate salts consist of an acetate anion plus a cation (a positively charged atom or molecule). The chemical formula for an acetate anion is [CH3CO2]- , usually written [CH3COO]- .
Source: General Chemistry Online!
Image: Wikimedia Foundation - Acetic Anion Balls 3-D
The chemical formula of acetate esters is much the same as that of the acetate ion. There is a very significant difference, however: acetate esters add an additional molecule, called a group or side chain. This group is denoted R. The chemical formula for esters, then, becomes CH3O2R.
For example, ethyl acetate is one widely known acetate ester. The chemical formula of ethyl acetate is CH3COOC2H5, so C2H5 is the side chain.
Source: JTBaker online MSDS for ethyl acetate.
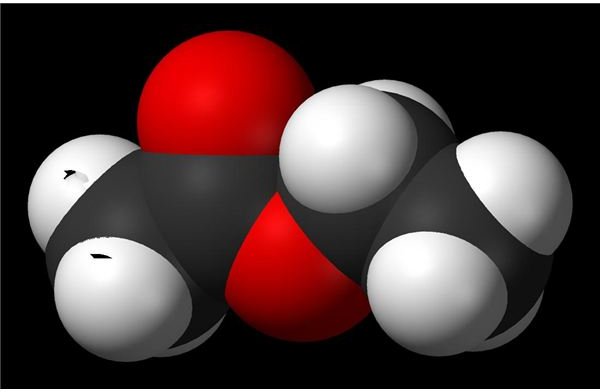
Image: Wikimedia Foundation - Ethyl Acetate 3D
Selected Industrial Uses of Acetate
One of the primary uses for acetate in industry is as a solvent. In this capacity, acetates are frequently used in paints, coatings, and various ink techniques. Polyvinyl alcohol is made from vinyl acetate (chemical formula CH3COOCH=CH2) and is used in a number of paints. Polyvinyl acetate is itself quite useful, being a popular type of wood glue.
Ethyl acetate is another popular type of acetate, with over 1 million pounds produced each year in the US alone. Ethyl acetate is used as a solvent in many applications, such as in paints, printing, varnishes, and in the laboratory. It is also used as a solvent in electroplating.
Source: Scorecard Chemical Profiles
Another common industrial acetate is cellulose acetate, one of the first synthetic fibers. Cellulose acetate has many uses, including in eyeglass frames, playing cards, and even diapers.
Source: AllAboutVision.com
Acetate salts also have a number of important industrial uses. Potassium acetate, for instance, is often used as a food preservative, while aluminium acetate is used as an antiseptic and as an antistringent.
Source: Nithyasri Chemicals
Summary
The chemical formula of acetate is, generally speaking, CH3O2 plus an additional group or side chain. There are two types of acetates: salts and esters. These different chemicals are very widely used in industry in a number of capacities, including solvents, food chemicals, and artificial fibers.