Facts About Absolute Zero
Introduction
Absolute zero temperature is the temperature at which no heat energy remains in a substance. This point of a substance is known as zero-point energy and this is due to the fact that the substance has its minimum energy at this point according to the quantum theory.
Some interesting and informative facts regarding absolute zero are given below.
First discussion about existence of Absolute Zero Temperature
The possibility of minimum temperature of substances was discussed by Robert Boyle for the first time. His 1665 New Experiments and Observations touching cold expressed the argument which is known as “primum frigidum.” Many ideas were introduced by scientists at that time. Some of them claimed that the condition of absolute temperature occurred in earth. Some of them said it occurred in water, and atmosphere was also considered as the place of occurrence of absolute temperature. However, their research met at a point, which explains that there exist some bodies or substances that have a different nature and they are cold nearer to absolute temperature. These substances can develop the same quality as the substances that come into their contact.
Who introduced Absolute Zero Temperature
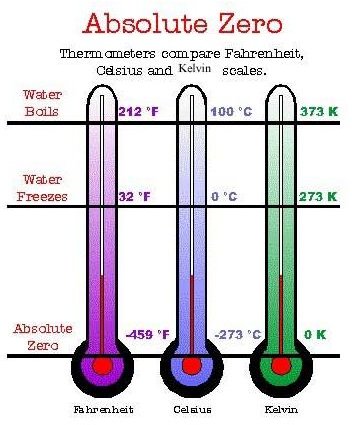
The scale of absolute temperature was developed by Lord Kelvin in 1848. The main property of this scale is that it does not depend upon the properties of a substance; however, the scale totally depends upon the laws of thermodynamics. The divisions of this scale are equivalent to the divisions in Celsius scale but the zero point of this scale shows the temperature -273.15°C and 100 °C is shown as 373.15 K.
Comparison of Absolute Temperature with Celsius, Rankin, and Fahrenheit scale
Absolute temperature is defined as exactly 0 K on the Kelvin scale, which is also known as absolute zero scale. It is equal to -273.15°C on the Celsius scale. It is also exactly equivalent to 0 °R on the Rankin scale and –459.67 °F on the Fahrenheit scale. These scales are also important thermodynamic temperature scales.
Effect of Absolute Temperature on entropy
Absolute temperature is a theoretical temperature at which the entropy of a substance reaches its minimum value, i.e., zero**.** The entropy of a body depends on the amount of heat transfer from the body; however, if the temperature of the substance is absolute zero, then it cannot transfer any heat, and the entropy will be zero if there is no heat transfer.
Practically, Absolute Zero temperature is not possible!
The occurrence of absolute temperature is only possible theoretically, but practically it is impossible. If we create absolute temperature anyhow; however, it’s impossible to measure it. This is because the measurement of temperature is based on the concept of energy flowing to the substance, and the substance will start gaining energy due to the flow of energy for measurement. Due to this, after gaining energy, the substance will not remain at absolute temperature anymore.
Only one condition to achieve Absolute Zero Temperature
It is described according to the laws of thermodynamics that a thermodynamic system can reach at absolute zero point only when it is entirely removed from the rest of the universe.
Equipment to achieve temperature close to Absolute Temperature
A substance cannot reach absolute zero temperature, but it is possible to achieve temperature very close to it by some newly developed techniques such as cryocoolers and Laser cooling.
Effect of achieving minimum temperature on a substance
At the temperature very close to absolute zero, most substances start showing some unusual properties such as superconductivity, superfluidity, and Bose-Einstein condensation.
Temperature of the Universe
According to the theory of cosmic microwave background radiation, the temperature of the universe is 2.73 K.
Record of lowest temperature of a substance
The current record of lowest temperature was achieved at 100 Pico Kelvin in 1999 by cooling a piece of rhodium.
Reference
Image: ffden-2.phys.uaf.edu-Comparison of Absolute Zero