Electrical Power from Coal Gassification
Introduction to Coal Gasification
With the world’s supply of hydrocarbons due to run out at the end of the century, we need to find an alternative to our use of oil and gas in our power plants.
There are abundant supplies of coal set to last centuries, but the combustion of pulverized coal to produce electricity is the biggest emitter of CO2 of the fossil fuels.
On the other hand Syngas, a product of coal gasification, can provide an alternative source of energy from coal, but only if the CO2 is captured and stored as in sequestration (see my articles on CCS) so that we have a lot less air pollution.
Now, in the power station if we combine CCS with an integrated gasification combined cycle (IGCC) then use the waste heat from the steam turbine condenser to run community heating (CHP) we could have a system of very high efficiency, up to 75%.
In this article we will start by taking a look at the coal gasification process and move onto the power station operation which uses the syngas to drive a gas turbine producing electricity to the national grid.
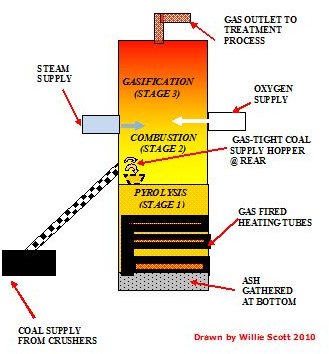
Gasification of Coal Process
The coal used for gasification is normally about 75 mm pieces which have been subjected to grinding or ball mills.
Gasification of the coal basically consists of loading these small uniform pieces by conveyor onto a moving grate located about a quarter of the way up the gasifier. The gasifier is a vertical pressure vessel in which the coal is subjected firstly to high temperature in virtually anaerobic conditions in the pyrolysis stage, producing tar, coke particles, and volatiles.
As the coke particles, tar, and volatiles rise up the gasifier, they are then subjected to further intensified heating in the combustion stage. In this second stage, oxygen and steam are injected into to the coke and volatiles, causing them to rapidly combust. This supplies more heat during the gasification stage, which is caused as it reacts with the carbon dioxide and steam. This reaction forms the gasses of hydrogen and carbon monoxide, occurring from about three quarters of the way up the gasifier to the top. This gas then exits at the top the gasifier, is cooled, scrubbed and filtered, with the end product being a synthetic gas suitable for use in a gas turbine.
The use of oxygen as opposed to ambient air will produce a purer gas having a higher calorific value.
However the oxygen has to be separated from the air, in an air separation unit (ASU). This procedure requires electrical energy, so it is a balance of these factors which will decide whether to use air or oxygen in the combustion stage of the gasification of coal.
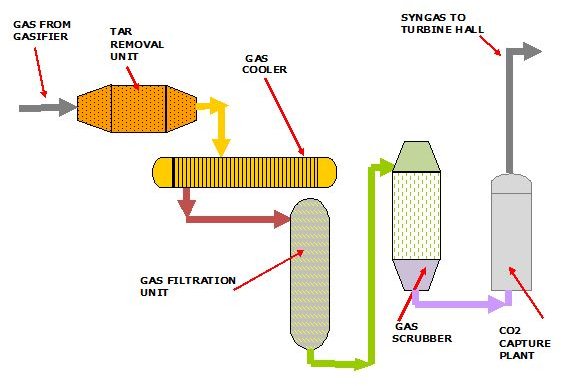
The Treatment of Syngas
The syngas exits the top of the coal gasifier and is passed to the gas treatment plant which consists of coal tar removal, gas water reactor and cooling, dust filtration, and SOx scrubbing processes.
Most of these residues can be further processed and sold to chemical and building industries.
After treatment, the gas then passes through the CO2 extraction plant where the CO2 is separated and stored, ready for transport to a long term storage area. Following this process the zero CO2 syngas is piped to the gas turbines in the turbine hall.
Sketches of gasifier and process system
Sample contents.