Use of Ionic Liquids for Synthesis and Deposition of Conductive Polymers
What is an Ionic Liquid?
An Ionic Liquid (IL) is a salt, like NaCl, in a liquid form. Ionic liquids consist of ions (Na+,Cl- ) instead of neutral atoms and can be used as solvents or electrolytes. Salts, in general, are found in a solid crystalline state but as soon as they reach their melting point, they become liquids. Certain salts like piridinium chloride C5H6N+·Cl−, can exist in a liquid form even at room temperature and are therefore called room temperature ionic liquids (RTIL).
History of Ionic Liquids
Ionic liquids have been known to scientists since the beginning of the 1900s. One of the first IL to synthesize was 1,3-dialkylimidazolium. These first ILs were not stable and inert to most organic compounds and therefore, their applications range was narrowed down. As soon as the first RTILs for organometallic catalysis were available in the middle of 1990s (e.g. hexafluorophosphate (BMI.PF6), they became popular again. The N,N-dialkyl imidazolium ILs are the most widely investigated today. At the same time, there is a variety of other promising low-melting salts researchers are focusing on, in order to produce efficient catalysts, solvents and reagents.
Synthesis and Deposition of Conductive Polymers with the aid of Ionic Liquids
Ionic liquids are suitable for the synthesis of conductive polymers, since they have a wide electrochemical window which ensures their excellent oxidative and reductive stability. This capability made them more popular in recent years than the conventional molecular solvents and electrolytes.
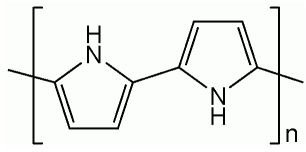
Conductive polymers such as polypyrrole, polythiophene, polyaniline etc. are organic polymers that conduct electricity, and are used as a replacement for silicon in a range of applications. These polymers can be easily synthesized by polymerization of monomers in the presence of molten salt. Since they have conjugated systems of electrons, they can be either reduced or oxidized by chemical or electrochemical processes. Their most popular applications include organic electronics, organic light emitting diodes (OLEDS), organic polymer solar cells (OPVS), fuel cells, etc.
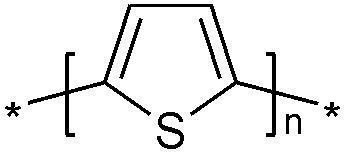
A polymer that has drawn attention due to its wide range of applications (conductor, electrode, and semiconductor) during the last few years is Polythiophene (Pth). It is usually synthesized by electrochemical oxidation of the thiophene monomer in an organic solution. Ionic liquids are excellent solvents for many organic compounds and 1-butyl-3-methylimidazoliumhexafluoro-phosphate ([BMIM]PF6) ionic liquid was reported to be an excellent solvent for Polythiophene (electrochemical synthesis of Pth).
Ionic liquids can also be used as a media for the electrochemical deposition of conducting polymers onto surfaces to produce, for example, electrochromic films for electronic display devices. Polypyrrole (Ppy) is considered to be suitable for such electrochromic applications. By applying a relatively high potential, the polymer can be deposited onto a metallic surface such as iron, without any significant overoxidation problems compared to the use of other depositing media.
Other General Applications of Ionic Liquids
The use of ILs as solvents for organic syntheses, catalysis, and deposition is only a sample of the various applications these materials have.
- Medical: liquid minerals such as ionic liquid calcium, cesium, potassium, selenium, sodium, sulfur, zinc, vanadium, germanium, iron, iodine, and cobalt are used for medicine purposes to better ensure the absorption of the minerals by the organism. Chiral ionic liquids (“left-handed” or “right-handed” molecular structured) are also used for pharmaceutical applications.
- Biological: drug delivery, biomass processing, biocides
- Electrochemical: batteries, solar panels, ion propulsion, fuel cells, electro-optics
- Physical: heat transfer, lubricants (e.g. Evionic ionic liquids) , RI matching, templates for the synthesis of nano-materials, magnetic ionic liquids (conducting, inflammable, non-volatile).
- Solutions: “dry” cleaning, coatings, household products, metal extraction, embalming, tissue preservation.
Ionic liquids have a very promising potential as far as applications are concerned. Although the conducting polymer research field is still in its infancy, the benefits of using ILs in this research are evident already. The stability of an electrochemically deposited film is much better when ionic liquids are used instead of organic solvents. Furthermore, the synthesis of conductive polymers with the aid of ILs is in the spotlight of various international research projects during the last years. Their aim is to develop these polymers in such a way, so that they may be produced in large quantities and therefore be available for commercial applications as mentioned (OLEDs, OPVs, etc.).
Sources:
- Ionic Liquids, Laboratory of Molecular Electrochemistry, Department of Chemistry I.F.M., Torino
- “Forming Conducting Polymers Utilising Room Temperature Ionic Liquids”, L. Astratine, D.I.T.
- “Synthesis of Conducting Polymers in Ionic Liquids”, J.Pringle, O.Ngamna, J.Chen, G.G.Wallace, M.Forsyth and D.R. MacFarlane, University of Wollongong, Australia
- “Ionic liquids for promising ion-conducting polymer materials of electrochemical devices”, Î.V. Chervakov, M.V. Burmistr, O.S. Sverdlikovs’ka, V.H. Shapka, Ukrainian State Chemical Technology University
- https://www.chemlin.de/
- https://www.floridaherbhouse.com/
- “Electrochemical Synthesis of Polythiophene in an Ionic Liquid”, Jia Hua SHI, Xun SUN, Chun He YANG, Qing Yu GAO, Yong Fang LI, Chinese Chemical Letters, 2002