Formaldehyde Resins used in Industry, Manufacturing, and Construction
Formaldehyde Resins as Engineering Materials
Do you know what makes fabrics crease-resistant? Furthermore, do you know how different layers of plywood are made to stick together?
While it is easy to answer that permanent adhesives are what cause various materials to stick together, or onto a particular surface, it is not very easy identifying the compounds that make up these adhesives. However, if you are familiar with formaldehyde, knowing the answers to these questions may not be that difficult at all.
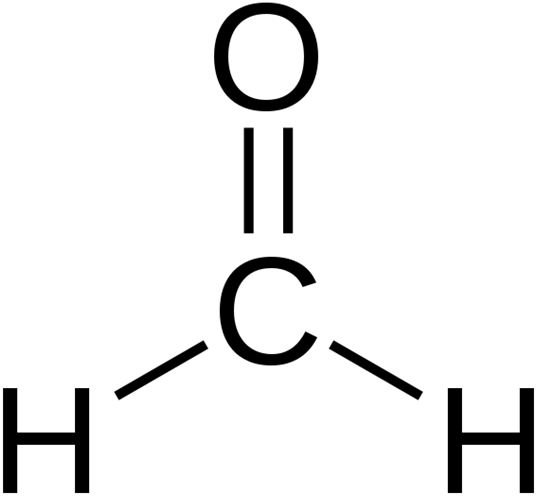
Fig.1 Formaldehyde (Chemical Composition)
Image. Wikepedia.com
Formaldehyde is an organic compound that, unlike many simple carbon compounds, can assume different forms . Because of this characteristic, formaldehyde is a common building block for the synthesis of more complex compounds. Formaldehyde assumes the chemical formula, CH2O, which basically means that it is generally composed of carbon, hydrogen, and oxygen atoms. The simplest form of formaldehyde is aldehyde, which is an important precursor to many other important compounds. Its molecular structure is a trigonal planar, and its related compounds are acetaldehyde, propionaldehyde, methanol, and formic acid.
While formaldehyde is generally a gas at room temperature, it can easily convert to other derivatives. On the other hand, it is also easily dissolved in water, with which its aqueous solution is known as formalin. Moreover, formaldehyde is extensively used in the production of polymers and other materials.
Although formaldehyde is known in various forms, it is more popularly known in its resin form, where it is most commonly used as a thermosetting resin or plastic. Some of the important products generated from formaldehyde are urea formaldehyde resin, phenol formaldehyde resin, melamine resin, polyoxymethylene plastics, and methylene disphenyl diisocyanate.
Urea Formaldehyde Resins
These are non-transparent thermosetting or plastic resins. Urea formaldehyde is also known as urea-methanal because of its overall structure and common synthesis pathway. Urea formaldehyde resins are generally used in adhesives, MDF, finishes, and molded objects. Moreover, these are used in gluing wood together, as in plywood. In addition, urea formaldehyde resins are also commonly used in the production of electrical appliances such as desk lamps.
Phenol Formaldehyde Resins
These resins include synthetic thermosetting resins that are obtained by the reaction of phenols with formaldehyde. These are probably the most widely used formaldehyde resins as these are used in various industries and engineering applications. Phenol formaldehyde resins are best known for the production of molded products such as laboratory countertops. These resins are also used as coatings and adhesives. In addition, paper phenolics are used in the manufacture of electrical components like household laminates and punch-through boards. The most popular trade name of phenolic formaldehyde is Bakelite.
Melamine Resin
Melamine resins are formed when melamine reacts with formaldehyde. These resins are generally hard thermosets which are used in permanent adhesives used in the manufacture of carpeting and plywood. Moreover, these are also used as additives in sanitary products like table napkins, facial tissue, and roll towels due to their wet-strength property. Furthermore, melamine resins are also foamed to make cast-into molded products and to make insulation.
Polyoxymethylene Plastics
Polyoxymethylene or POM is more popular under the trade name, Delrin. This is an engineering plastic which is most commonly used as a metal substitute. This is so because POM is capable of operating at temperatures above 90 degrees Celsius. Some of the distinguishing characteristics of polyoxymethylene include low-friction, wear resistance, and good physical and processing properties. Delrin is used in making combination wheels in high-security locks. Moreover, it is also used in making diving equipment because it is lightweight and has low porosity to gas under pressure.
On the other hand, polyoxymethylene is also known as polyacetal, polytrioxane, polyformaldehyde, and acetal resin. Its important copolymers are sold under the trade names Celcon, Hostaform, Ultraform, and Kepital.
Methylene Disphenyl Diisocyanate
This is a formaldehyde derivative which is an important component in making polyurethane paints and foams. Most often abbreviated as MDI, this compound exists in three isomers namely 2.2’-MDI, 2.4’-MDI, and 4-4’-MDI. Basically, the major application of MDI is in the production of rigid polyurethane, although 4.4’-MDI is also used as an industrial adhesive. Polyurethane foams made from MDI possess good thermal insulation property and are used in most freezers and refrigerators worldwide.
References:
1. Http://tripatlas.com/Formaldehyde
2. Randall, David; Lee, Steve (2002). The Polyurethanes Book. New York: Wiley. ISBN 0-470-85041-8
3. Christian Six and Frank Richter “Isocyanates, Organic” in Ullmann’s Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a14_611