Parameters Involved during Carbon Steel Heat Treatment Procedures
Carbon steel is attributed with its properties due to the presence of carbon in it. Steels revealing other properties may be composed of other elements and are called alloys. The influence of carbon may be better understood only after studying pure iron.
Pure iron is highly ductile with great levels of tensile strength, around 18 tons per square inch.
When carbon is added to iron, its tensile capability, hardness and brittleness increases, but decreases its ductility.
However the above enhancements are dependant on the carbon content which must be limited to 1.7% at normal heat treatment. Beyond this level the excess carbon goes into Free State or forms graphite which has very low tensile strength. The carbon level in plain carbon steel should never increase above 1.5%.
When we heat and cool steel its atomic structure changes which results in the change of its physical properties to significant extents. Particularly the process results in hardening of steel and finds many important applications in different fields. The process of heating and cooling steel for acquiring the desired results is called heat treatment of steel. However it doesn’t guarantee ideal or exact desired changes in steel and the manufacturer may need to be satisfied with compromised results. For example softer steel though may not be hard, it tolerates bending stress to an extent and therefore is tough, but if it’s hardened, it may become brittle and lose its resistance against bending and other forms of tensile stresses.
Carbon steel heat treatment involves some important parameters which are discussed as follows:
Structure Of Steel
Carbon steel is primarily made up of pure iron (ferrite) and also consists of chemical compound called iron carbide made by introducing carbon in iron.
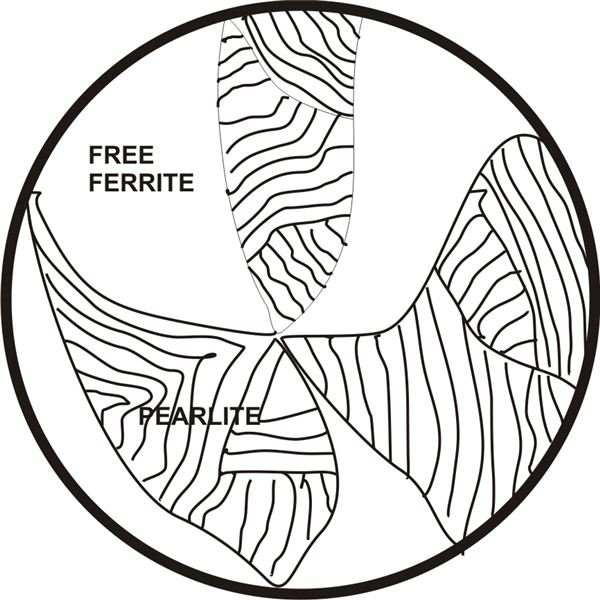
A cross sectional view of low carbon steel under a microscope shows it to be laminated in structure (pearlite), engulfed with free ferrite. As carbon content is increased, the pearlite to ferrite proportion also increases until the carbon content reaches 0.87 percent when steel completely turns into pearlite.
On further increase of carbon content (above 0.87%), a microscopic slide uncovers pearlite surrounded with cementite.
Property wise ferrite is recognized to be soft and ductile, not very strong. Pearlite is tough and strong, it’s also soft enough and workable. Cementite though very hard, is brittle.
The above analysis shows that as carbon content is increased in steel up to 0.87%, steel becomes tougher and stronger, but beyond this level, the increase in cementite content makes steel much harder and gradually brittle.
Critical Point
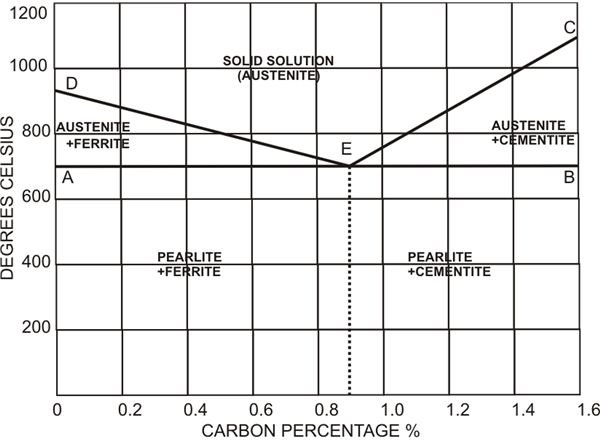
Heating of steel does not produce a constant rate of increase of its temperature but rather through an uneven flow. Initially the temperature may show a steady rise after which it may stop absorbing heat for a moment and after a short period of temporary halt the temperature again starts rising normally. During this short pause, the metal absorbs heat which instead of heating further, initiates certain transformations in the metal. The temperature at which this change occurs is termed as the “critical” or “arrest” points.
The diagram shows equilibrium conditions, where the critical points are illustrated over an increasing temperature. The line AEB indicates lower critical points while the line DEC the higher critical points.
However it is important to remember that though steels may vary in their carbon compositions, their lower critical point is the same = 730 degree Celsius.
At this temperature pearlite ceases to exist and laminae of other compositions like ferrite and cementite dissolve to form a solid structure called austenite, which shows non-magnetic properties.
The proportion of pearlitee and cementite may be such that the mixture produces a solid solution at lower temperatures. The solidness may be more than that obtained with other mixtures of steel, like pearlite and eutectoid.
On heating steel a little above the critical point temperature and then cooling suddenly produces no effect over its structural form. The quick cooling restricts the formation of pearlite, however austenite may change into a different atomic structure called “Martensite”, which exhibits extreme hardness and brittleness.
In case the cooling is not done drastically, then the procedure may transform austenite into another form called Troostite, which is not so hard but is very tough.
If the cooling procedure is made at a further slower rate then the transformation results “Sorbite”, a finely grained, strong and ductile structure.
The above discussion shows how the degree of hardness may be varied by observing different rates of heating and cooling.
Image: By Karann
Methods of Heating Steel
Heat treatment method of carbon steel is one of the critical parameters that must be observed carefully for benefitting optimum results. Along with the temperature limit which must be carefully regulated, the heating procedure should be gradual and uniform.
Heating equipment which guarantees rigid heat control is recommended.
Muffle furnaces which are operated using oil, gas or electricity become more preferable and are much efficient. These are able to generate high degrees of temperatures without the hazards of over heating or damaging the metal which is being heat treated.
Never opt for a blacksmith’s furnace because they are not suitable for the purpose, as they do not support controlled and uniform heat outputs and may overheat the metal until it burns off.
In case a blacksmith’s furnace is employed, make sure the job is heated in a steel tube and not directly inside the flame.
Ideally oil or gas heated bath containing molten alloys of low melting points or molten salts become very suitable for steel heat treatment. This type of heating promises a well regulated heating of steel and also supports heating of irregular shaped jobs. The salts used in the bath may be altered for getting different levels of temperature.
References