Boyle's, Charles & Gay-Lussac's Law from Ideal Gas Law
The Ideal Gas Law and Avogadro’s Number
In terms of Avogadro’s number NA, we can write the number of moles n as:
- n = N/NA
where N represents the total number of molecules in a gas and NA = 6.02 x 1023 molecules/mole. We found in part one that the ideal gas law may be written as:
- PV = nRT
Substituting 1 into 2 yields:
- PV = N/NART
We can simplify this equation even further with the use of Boltzmann’s contant. Boltzmann’s constant is defined as:
- k = R/NA
which is 1.38 x 10-23 J/K in SI units. Then equation 3 becomes:
- PV = NkT
This is another standard way of writing the ideal gas equation.
Another Form
It is not always necessary to use the number of molecules or Boltzmann’s constant.
In this case, the ideal gas law is also commonly written as:
- P1V1 / T1 = P2V2 / T2
where P1,V1, and T1 are the original values of the gas, while P2,V2, and T2 represent its final values.
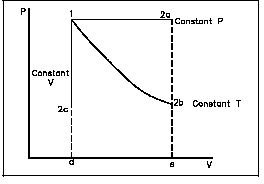
We can use equation 6 to derive Boyle’s, Charles’, and Gay-Lussac’s Laws. We do this by considering isothermal, isobaric, and isochoric thermodynamic processes.
Derivation of Boyle’s Law
Isothermal
Isothermal means that the temperature is constant. When we do this, T1 = T2 = T, so:
- P1V1 / T = P2V2 / T
The Ts cancel, and we are left with Boyle’s Law P1V1 = P2V2.
Derivation of Charles’ Law
Isobaric
Isobaric means that the pressure is constant, so P1 = P2 = P, giving us:
- PV1 / T1 = PV2 / T2
With the Ps canceling, we are left with Charles’ Law V1/T1 = V2/T2.
Derivation of Gay-Lussac’s Law
Isochoric
Finally, isochoric means the volume is constant, such that V1 = V2 = V, and thus we have:
- P1V / T1 = P2V / T2
The Vs cancel, giving us Gay-Lussac’s Law P1/T1 = P2/T2.
References
Physics for Scientists and Engineers by Douglas Giancoli
Fundamentals of Physics by Halliday, Resnick, and Walker
Image Credits
Ideal gas law from www.EngineersEdge.com
Resources
Comprehensive List of the Various Forms of the Ideal Gas Law
This post is part of the series: Introduction to the Ideal Gas Law
This series gives an elementary, non-calculus based introduction to the ideal gas law, including an account of its origins and how to use it to derive Boyle’s, Charles’, and Gay-Lussac’s Laws.