Anammox Wastwater Treatment instead of Nitrification and Denitrification
Ammonia Nitrogen in Wastewater and the Need for Treatment
It is helpful to have an understanding of nitrogen in wastewater and the nitrogen cycle in order to understand the innovation offered by anammox wastewater treatment. Some background is given here. For more details see “Easy to Understand Nitrogen Cycle Diagram.” When waste organic matter is decomposed in nature or in a wastewater treatment plant, the nitrogen in proteins is released as ammonia nitrogen (either dissolved ammonia gas, NH3, or ammonium ion, NH4+, depending upon the pH). This release of ammonia nitrogen from protein is one step in the nitrogen cycle. The next normal step in the nitrogen cycle is oxidation of the ammonia nitrogen to nitrite ion (NO2-) by the appropriate aerobic bacteria and then oxidation of the nitrite ion to nitrate ion (NO3-) by another variety of aerobic bacteria. These oxidation processes taken together are called nitrification and require oxygen in order to take place. If wastewater effluent containing ammonia nitrogen is discharged to a river, lake, or stream, nitrification will take place there, exerting a biochemical oxygen demand (BOD), and using up dissolved oxygen from the water, thus affecting aquatic life. This is the reason that nitrification is required of most wastewater treatment plants.
Nitrification and Denitrification in the Traditional Nitrogen Removal Process
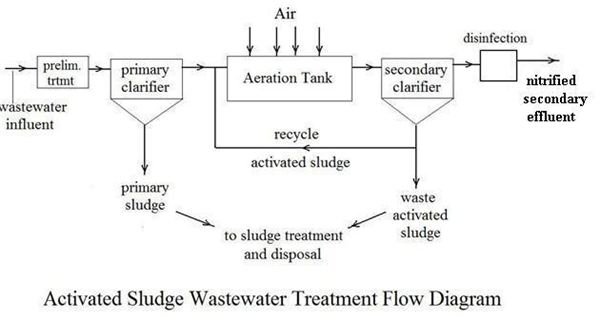
The nitrification that is required of most secondary wastewater treatment plants can be accomplished in a typical activated sludge wastewater treatment process by ensuring that there is adequate aeration to provide the necessary oxygen and by maintaining a long enough sludge retention time so that the slower growing nitrifying bacteria aren’t washed out of the system. With these steps the bacterial oxidation of ammonia nitrogen to nitrate (nitrification) will take place along with the bacterial oxidation of hydrocarbon BOD, as shown in the process flow diagram at the left. Nitrification, however, doesn’t remove the nitrogen from wastewater. It simply removes the oxygen demand, by converting ammonia nitrogen to nitrate nitrogen. If discharge of wastewater treatment effluent containing nitrate is a problem for the receiving body of water, then another process, denitrification, is also required.
Denitrification is another bacterial process, but it takes place in an
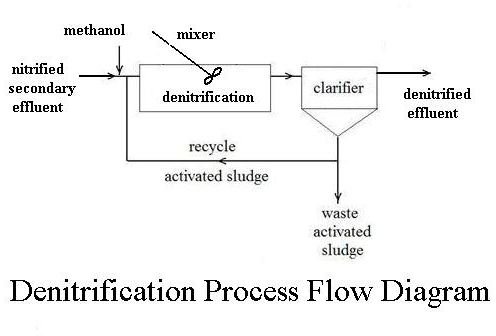
anaerobic environment, that is in the absence of oxygen. The appropriate bacterial culture, in an anaerobic environment, will convert nitrate (NO3-) to nitrous oxide (N2O) and then to nitrogen gas (N2). Nitrogen gas is an ideal end product. It is simply given off to the atmosphere, where it is already the major component of air. The denitrification process doesn’t require aeration, but it does require a separate tank and mixer, and a carbon source (typically methanol) must be fed in for the denitrifying bacteria, as shown in the flow diagram at the right.
Background on Anammox Bacteria and Their Discovery
The discovery of the type of bacteria now commonly called anammox bacteria is quite recent. In the mid-1990s anaerobic oxidation of ammonium ion was observed in a wastewater denitrifying pilot plant1. Subsequently the bacteria commonly called anammox bacteria were isolated and identified2. At about that time, it was also discovered that anammox bacteria are responsible for the conversion of a great deal of ammonia and nitrite nitrogen to nitrogen gas in our oceans and seas. The characteristic of anammox bacteria that is of particular interest for wastewater treatment is their ability to convert ammonium ion and nitrite ion into nitrogen gas in an anaerobic environment.
An Alternative Nitrogen Removal Process Using Anammox Wastewater Treatment
Instead of oxidizing all of the ammonia nitrogen in a wastewater flow to nitrate and then denitrifying that nitrate nitrogen to nitrogen gas, as described above, anammox bacteria present an interesting alternative. If only half of the ammonia nitrogen in a wastewater flow were oxidized just to nitrite, then the remaining ammonia nitrogen and the nitrite nitrogen that has been formed could be converted to nitrogen gas by anammox bacteria in an anaerobic (absence of oxygen) environment.
It turns out that this can be accomplished in a practical manner resulting in the need for less than half as much oxygen (resulting in less power cost for aeration) and less excess sludge for disposal, in comparison with the traditional nitrification/denitrification processes for removal of nitrogen from wastewater. A key factor that facilitates carrying out this process is the fact that the bacteria that oxidize nitrite to nitrate (nitrobacter) grow much more slowly than the bacteria that oxidize ammonia to nitrite (nitrosomonas) and the bacteria that oxidize carbon and hydrogen containing organic matter to carbon dioxide and water. Thus maintaining the solids retention time relatively low (keeping the microorganisms in the system for only a short time) allows the oxidation of organic carbon BOD and ammonia to nitrite, but doesn’t allow the population of nitrobacter to build up to a level that allows oxidation of nitrite to nitrate. If the flow then goes to an anaerobic tank with a culture of anammox bacteria, the anammox reaction will take place converting the incoming nitrite and ammonia nitrogen to nitrogen gas.
Several alternative ways of carrying out these processes are under investigation and have been reported on3,4,5,6. At least three wastewater treatment systems using anammox wastewater treatment are currently in use.7
References Cited
1. FEMS Microbiol. Ecol. 16, 177–183, Mulder, A., Van de Graaf, A.A., Robertson, L.A. and Kuenen, J.G. (1995)
2. (1999) Nature (London) 400, 446–449, van de Pas-Schoonen, K.T., Webb, R.I., Kuenen, J.G. and Jetten, M.S.M.Strous, M., Fuerst, J.A., Kramer, E.H.M., Logemann, S., Muyzer, G.,
3. FEMS Microbiol. Lett. 218, 339–344Sliekers, A.O., Third, K., Abma, W., Kuenen, J.G. and Jetten, M.S.M. (2003)
4. Biotechnol. Bioeng. 77, 266–277Hao, X., Heijnen, J.J. and van Loosdrecht, M.C.M. (2002)
5. https://www.pars-treatment.com/news/view.asp?newsID=88